How to Produce Trans-Uranic Elements By Particle Bombardment

Uranium has the highest atomic number of any naturally occurring element and was, for a long time, placed at the end of the periodic classification of element. Trans-uranic elements have, however, now been prepared by nuclear reactions.
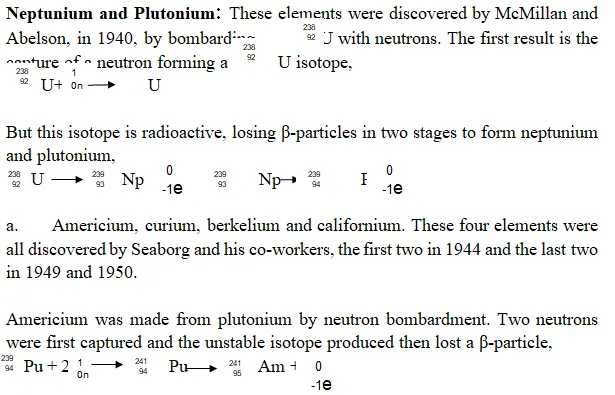
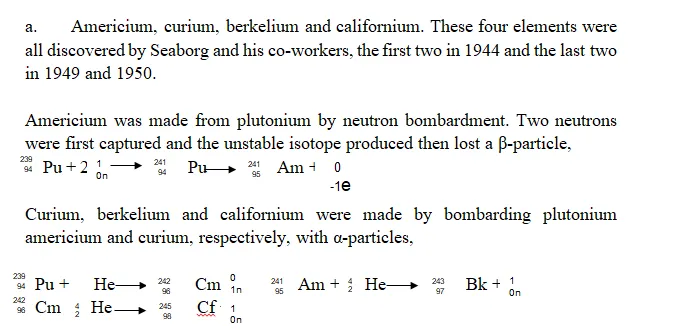
Examples of Trans-uranic Elements: The Trans-Uranic elements include, Plutonium, Neptunium, Americium, Curium, Berkelium, Californium, Einsteinium, Mendelevium, Nobelium and Lawrencium, Rutherfordium, Dubnium
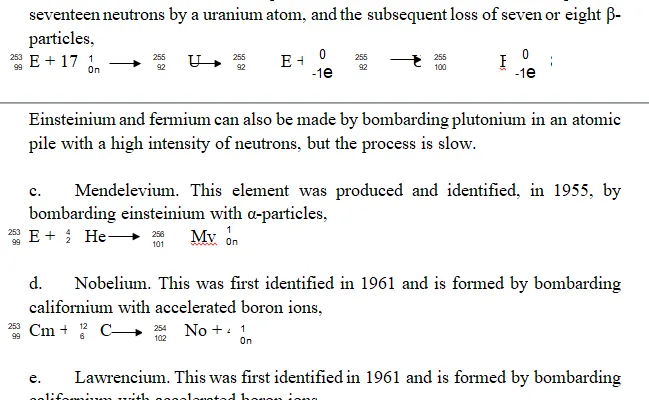
californium with accelerated boron ions.
Nuclear Stability: An atomic nucleus is made up of a number of protons equal to its atomic number, plus a sufficient number of neutrons to give the necessary mass number. The protons and neutrons in a nucleus are called nucleons.
Because the nucleus of an isotope must contain a whole number of protons and neutrons it was, for a while, thought that isotopic masses, i.e. the relative atomic masses of isotopes, might all be whole numbers. This revived a hypothesis first suggested by Prout, in 1815, in which he supposed that every atom was made up of hydrogen atoms.
Careful measurement, by mass spectrographic methods, show, however, that relative isotopic masses, on the 12C scale, are not exactly whole numbers. Some illustrative figures are given below
1H 1.007825 4H 4.00260 12C 12.00000
2H 2.01409 7Li 7.01600 35Cl 34.96885
![]()
These figures show how much heavier one atom of a particular isotope is than t of an atom of 12C. The actual mass of a 12C atom is readily obtained, for 12 gramme of the 12C, therefore, has a mass of 12/6.02252 x 1023 atom. One atom of 12C, therefore, has a mass of 12/6.02252 x 1023 gramme, and of this is 1/6.02252 x 1023, 1.66043 x 10-27 kg. this mass is often referred to as an atomic mass unit, the actual mass of any one atom being equal to its relative e isotopic mass multiplied by the value of the atomic mass unit.
For an isotope, the nearest whole number to its is known as the mass number. The slight difference between the actual relative isotopic mass and the mass number, known as the mass defect, is due to the fact that the masses of protons and neutron in a nucleus are not strictly additive because some part of their mass is converted into energy. This energy is the binding energy which holds the nucleus together; the greater it is, the more stable will the nucleus be.
For deuterium, with a simple nucleus of one proton and one neutron, the figures are as follows. The relative isotopic mass is 2.01409, giving an actual mass of 3.4426 x 10-27 kg. The mass of the nucleus will be 3.34426 x 10-27 kg. the deuterium nucleus consists of one proton of mass 1.67252 x 10-27 kg, and one neutron of mass 1.67482 x 10-27 kg, giving an additive mass of 3.34734 x 10-27kg.
The mass converted into energy, for a deuterium nucleus, to be formed, must, therefore, be (3.34734-3.34335) x 10-27 kg, i.e. 0.00399 x 10-27 kg. This is equivalent to a bonding energy of 3.583 x 10-13 joule or 2.238 MeV
In general, for a nucleus containing Z protons, and with a mass number of A, the number of neutrons will be (A-Z). If the mass of the proton is p, that of the neutron n, and that of the actual nucleus M, the mass converted into energy will be given by
Zp+ (A-Z)n-M
And this can be converted into energy units as explained below.
Note on units. When mass is converted into energy, the two are related by the equation
E = m x c2
(in joules) (in kilograms) (c in metres per second)
The value of c2 is approximately 8.99 x 1016 joule of energy
One electron volt (1 eV), which is the energy acquired or lost by a particle of unit electronic charge on passing through a potential difference of 1 volt, is equal to 1.602 x 10-19 joule, so that
1kg of mass = (8.99 x 1016)/(1.602 x 10-19)eV
=5.613 x 1035 eV
=9.613 x 1029 MeV
The unit of atomic mass is 1.66043 x 10-27 kg so that
1 atomic mass unit (amu) = 8.99 x 1016 x 1.66043 x 10-27J
= 1.494 x 10-10 J
= 9.328 x 108 eV
= 932.8 MeV
The figures given here are only approximate to show the relationship between the various units.



Trans-uranic elements have found applications in nuclear physics and chemistry. These elements are often deployed in the production of nuclear materials and explosives. The study of trans-uranic elements continues to open new frontiers in nuclear power generation and studies of climate change.